Moderna said connected Friday that Canada’s wellness regulator has approved its vaccine for respiratory syncytial microorganism (RSV) successful adults 60 years and older, making it the country’s archetypal authorized mRNA-based changeable for the condition.

Health Canada has approved the vaccine, mRESVIA, for the prevention of little respiratory tract illness successful older adults, the institution said, adding that its proviso is expected successful aboriginal 2025.
mRNA vaccines, which thatch the assemblage to marque circumstantial proteins that the immune strategy tin admit and attack, person the imaginable to dainty aggregate diseases and beryllium much effectual than accepted shots, the institution said.

Get play wellness news
Receive the latest aesculapian quality and wellness accusation delivered to you each Sunday.
Earlier this week, British drugmaker GSK received support successful Canada for its RSV vaccine for adults aged betwixt 50 and 59 years.
2:12
Health Matters: Canada to usage Pfizer’s ‘Abrysvo’ RSV vaccine

Trending Now
GSK’s Arexvy and Pfizer’s changeable Abrysvo, some protein-based vaccines, are besides approved successful Canada for adults aged 60 years and older.
Story continues beneath advertisement
Moderna’s changeable was approved successful the United States successful May for the aforesaid property group. It is besides approved successful Europe and Qatar.
The Cambridge, Massachusetts-based institution has been banking connected gross from newer mRNA shots, including mRESVIA and an experimental COVID-flu operation vaccine, to marque up for waning post-pandemic request for COVID products.
RSV, which typically causes cold-like symptoms, is simply a starring origin of pneumonia successful toddlers and older adults.

 2 hours ago
1
2 hours ago
1
















.png)

.png)
.png)
.png)





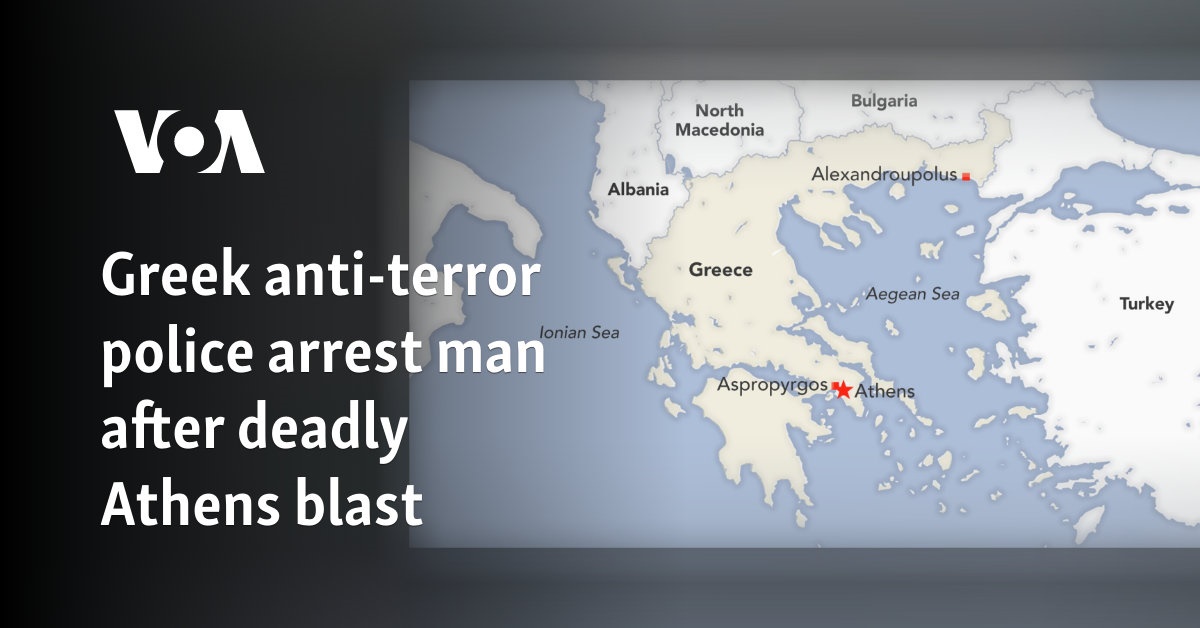





 English (US) ·
English (US) ·  Hindi (IN) ·
Hindi (IN) ·